Projects
Mechanisms of xylem cavitation and its repair
Evolution and ecology of xylem function
Hydraulic architecture and metabolic scaling theory
Modeling the soil-plant-atmosphere continuum
Mechanisms of xylem cavitation and its repair
Xylem sap is typically under negative pressure and hence in a metastable liquid state. It is vulnerable to cavitation, which is the abrupt phase change from metastable liquid to vapor. Cavitation is triggered by a nucleating agent, which in plants appears to be gas bubbles over a critical size. Cavitation creates a vapor bubble which relaxes the surrounding negative sap pressure to atmospheric pressure. As long as the sap in adjacent conduits remains under negative pressure, water is pulled from the cavitated vessel and the vapor bubble expands to fill the cavitated conduit. The gas phase would expand beyond the conduit if it were not for the inter-connecting pits which arrest the gas-water meniscus and seal off the vapor-filled conduit. As air diffuses into the vapor from surrounding tissue, the gas pressure rises to atmospheric and the conduit is fully "embolized".
Cavitation can occur from both freeze-thaw cycles and water stress, and there are interesting interactions between the two. Learning how gas-bubble formation arises under these circumstances is critical to understanding how species have evolved different environmental tolerances, and the inevitable trade-offs involved. As the graphic shows, we have a good model for how cavitation occurs. In the upper freezing situation, bubbles are formed in situ, and potentially nucleate cavitation during the thaw phase. It is possible that cavitation can occur during the freezing process itself, although we have not observed this in our experiments (Pittermann & Sperry 2006, PDF). Experiments have demonstrated a tight link between increasing conduit diameter and vulnerability to freeze-thaw cavitation (Davis et al. 1999, PDF), which makes sense if bigger conduits produce larger bubbles. However, this relationship is complicated by xylem pressure (Pittermann & Sperry 2006, PDF). Some species also show a puzzling response to the freezing temperature, with lower ice temperatures producing more embolism (Pockman & Sperry 1997, PDF). There is more to learn!
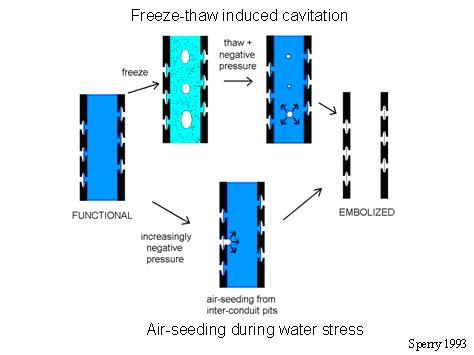
Mechanism of cavitation by freeze-thaw cycles (upper) and water stress (lower).
Cavitation by water stress (lower process in picture) appears to occur by air being pulled into the water-filled conduits through pits connecting with already embolized conduits. This "air-seeding" process occurs at very different pressures in different species, presumably because of differences in pit structure. However, discovering what those differences are has proven to be difficult (Sperry et al. 2006, PDF).
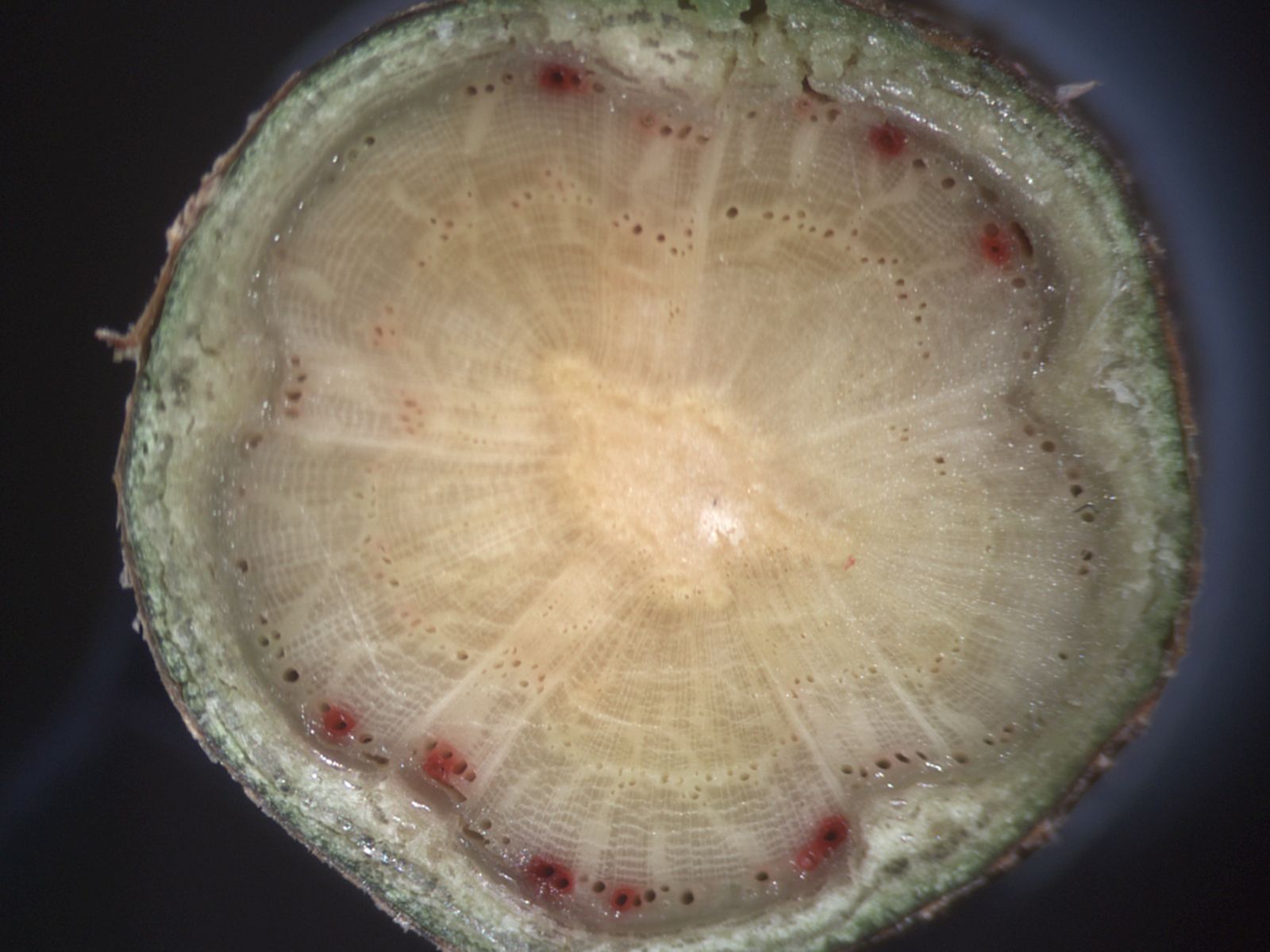
Our recent work on angiosperms highlights the important role that probability plays in cavitation by water stress. It only takes one "leaky" pit out of thousands that link angiosperm vessels to cause air-seeding, and although such pits are rare, just how rare they are (as well as how many pits there are per vessel) has a major influence on vulnerability to cavitation (Christman et al. 2009, PDF). Of particular interest are the large vessels in ring-porous trees and lianas because they may raise the risk of cavitation. Studying these vessels with typical destructive methods can be problematic, and have recently completed an MRI study of a ring-porous oak (see picture) which indicates that at least some of these large vessels can be remarkably resistant to cavitation…something we are now studying at the pit level. Conifer tracheids have pits with a different sealing-and-failing mechanism (Sperry & Tyree 1990, PDF), and we hope to determine the role of probability on air-seeding in conifers and their vessel-bearing gnetophyte relatives in the near future.
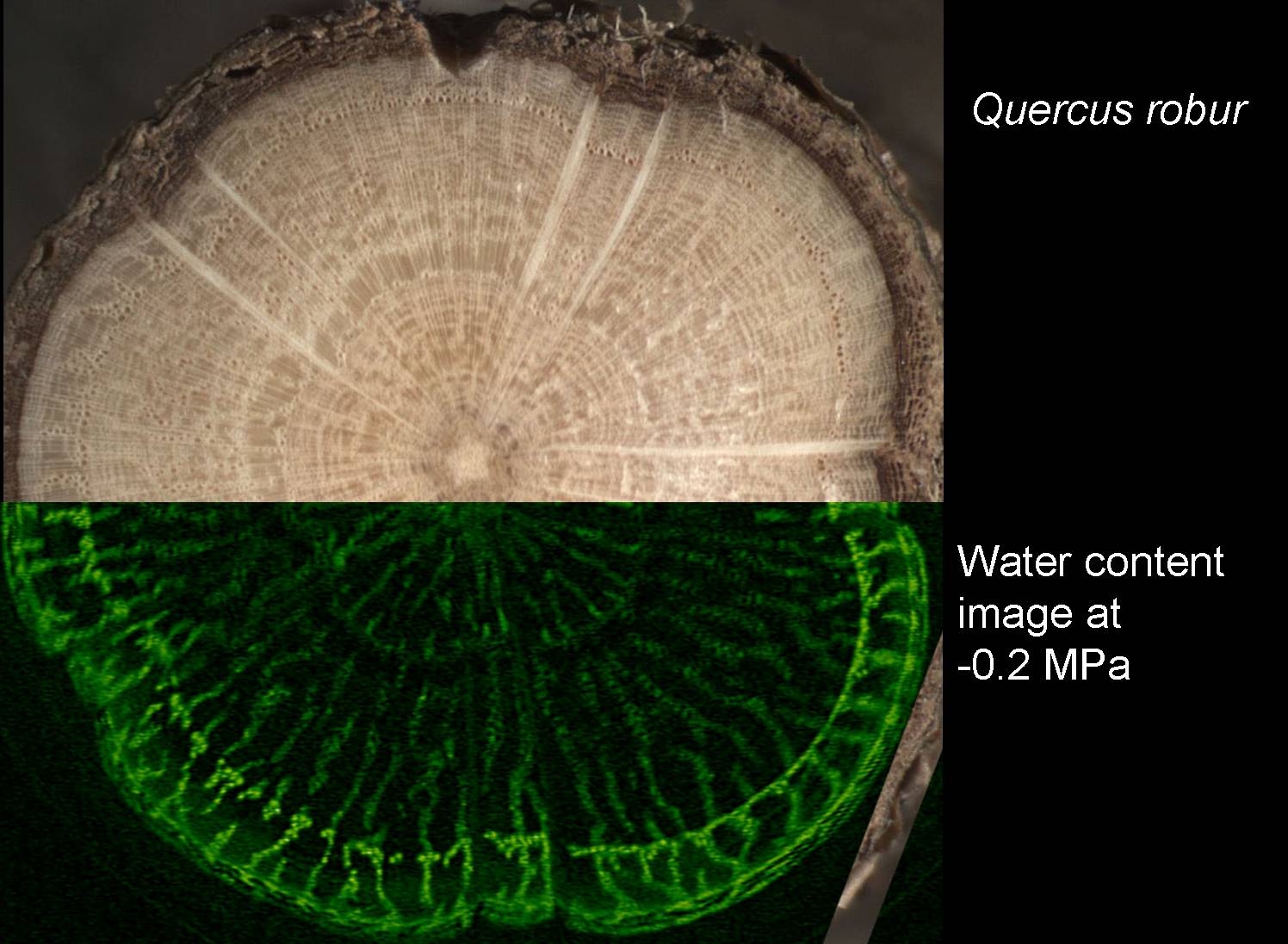
Cross section of oak imaged in microscope (upper) and by MRI (lower).
Once vessels or tracheids become embolized, some species are able to restore them to function by refilling them with sap. Years ago we extensively documented this process with regards to reversal of winter-time embolism via spring root pressures (Sperry et al. 1988, PDF; 1987, PDF; Sperry & Sullivan 1992, PDF). Failure of this refilling can lead to crown dieback. More recently, we and others have seen it occur in the absence of any positive bulk xylem pressure (Hacke & Sperry 2003, PDF; Stiller et al. 2003, PDF; Taneda & Sperry 2008, PDF). How this can happen is one of the biggest mysteries in plant hydraulics. Needless to say, we would like to figure this one out!
Evolution and ecology of xylem function
Secondary xylem (wood) performs many functions, but chief among them is long-distance transport and mechanical support of the canopy. Wood structure is amazingly diverse, and the meaning of this fascinating diversity begs for adaptive and evolutionary explanations. Wood anatomists, Sherwin Carlquist most notably, have carefully logged this diversity over the years and written books of adaptive hypotheses. But there are few or no experiments in much of this literature, and the reasoning is based on correlations and intuition. It's up to labs like ours which do comparative xylem physiology to test these and other inspired hypotheses of structure-function. As my advisor Martin Zimmermann once wrote…"what a rich field of investigation lay ahead!"
Three inter-related studies illustrate the trajectory we are following in this area. The first study was a comparison of the inter-conduit pits between angiosperm vessels and gymnosperm tracheids. It turned out that the different torus-margo structure of conifer pit membrane (See Picture) was 60x more efficient at water conduction than the homogenous pit membranes between angiosperm vessels, and equally as safe against air-seeding. The more efficient pits of conifers compensates for the shorter length of the single-celled tracheid. As a result, a single-celled tracheid has approximately the same conducting capacity as a multi-cellular vessel of the same diameter. We concluded that the evolution of these sophisticated micro-valves in gymnosperms was crucial to the success of this lineage, and helps conifers compete effectively for water with angiosperms (Pittermann et al. 2005, PDF).
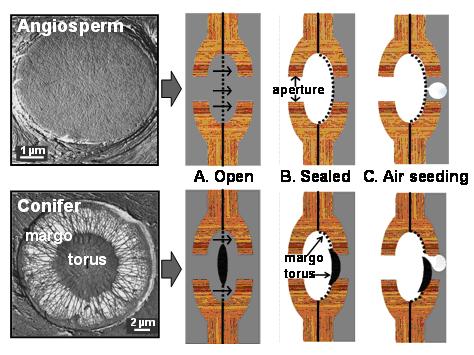
Contrasting structure and function of angiosperm and conifer inter-conduit pits.
The torus-margo study led us to investigate the consequences of vessel evolution in angiosperms, which produced some surprises (Hacke et al. 2007, PDF; Sperry et al. 2007, PDF. It turned out that vessel-bearing wood of basal angiosperms was statistically no more conductive on a sapwood basis than vesseless angiosperm wood, and the vessel-bearing taxa were more vulnerable to cavitation. The understory wet-forest habitat of the two groups was similar, so the data suggest that there was no hydraulic advantage to evolving vessels, and considerable risk was involved! The original advantage of vessels was probably that they evolved in concert with heteroxylous wood which has increased storage capacity and mechanical diversity. Efficient and safe vessels apparently required more modifications that came later, mostly in more late-diverging lineages. These modifications were to the perforation plate (scalariform to simple), vessel length (short to long) and pit membranes (more porous to less porous on average, so less vulnerable to air-seeding).
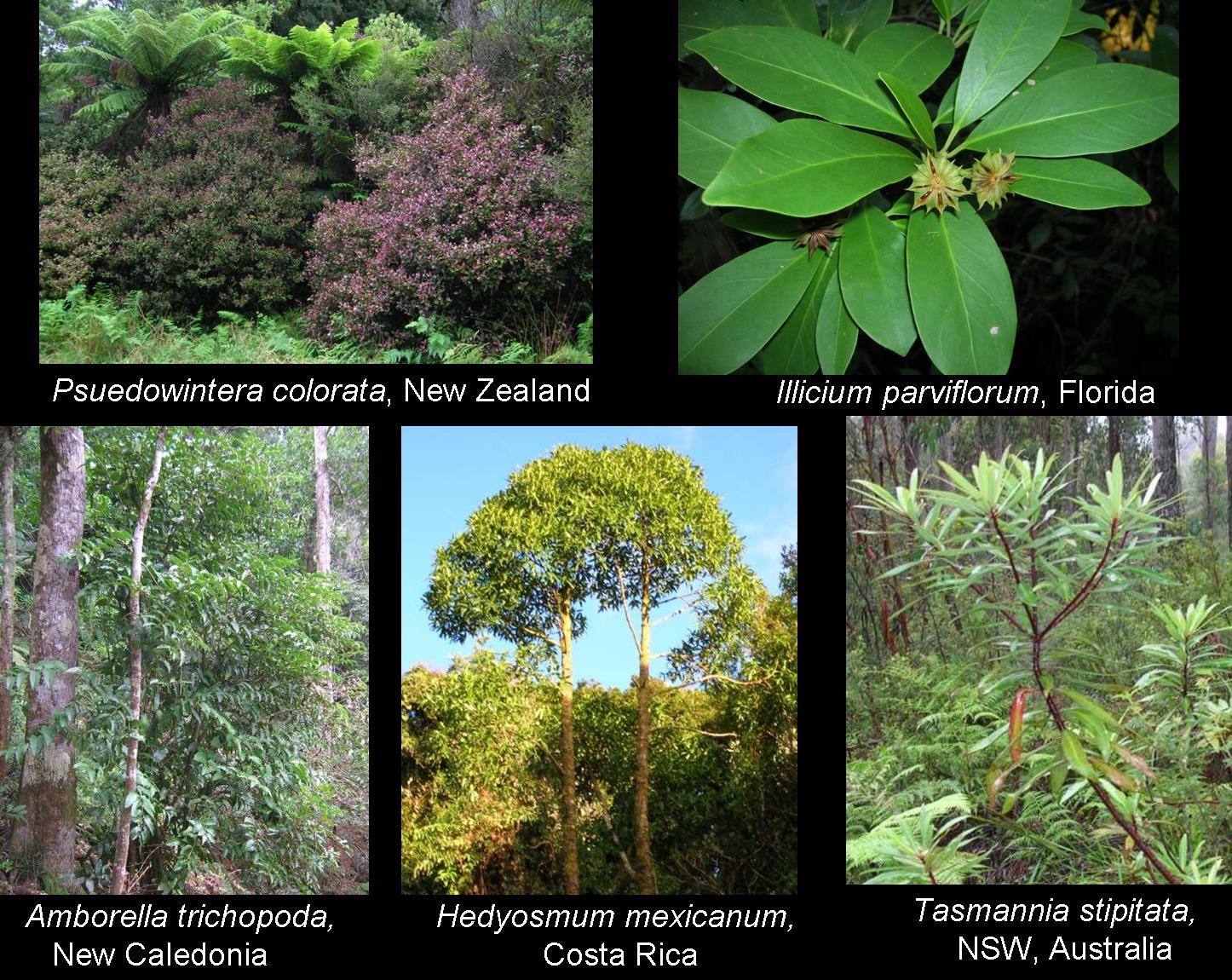
Some of the basal angiosperms, with and without vessels, collected for the comparative study.
A very recent project continues the thread by quantifying the hydraulic advantage of perforation plate evolution (Christman et al. in review). Perforation plates are the openings between individual vessel members. "Primitive" plates retain the scalariform (repeated slit) structure of inter-tracheid pitting in angiosperm tracheids, but of course the pit membrane is entirely or partially lost. The most derived plates are "simple" in which there is a single large circular opening. The text-book hypothesis is that this transition increased conducting capacity…but by how much? Computer models suggest the gain is trivial…on the order of 5-9%. Post-doc Maggie Christman mastered the single-vessel method (See picture) and measured numerous species with different morphology. As expected, simple plated lumens conformed closely to the Hagen-Poiseuille prediction for ideal capillary tubes…the best a xylem conduit can do. But scalariform species had only half the Hagen-Poiseuille capacity…indicating a capacity gain of 100% with the elimination of scalariform plates. It turns out that the earlier computer models were based on a limited range of plate morphologies, and we think that the high plate resistances we've measured are probably the rule rather than the exception.
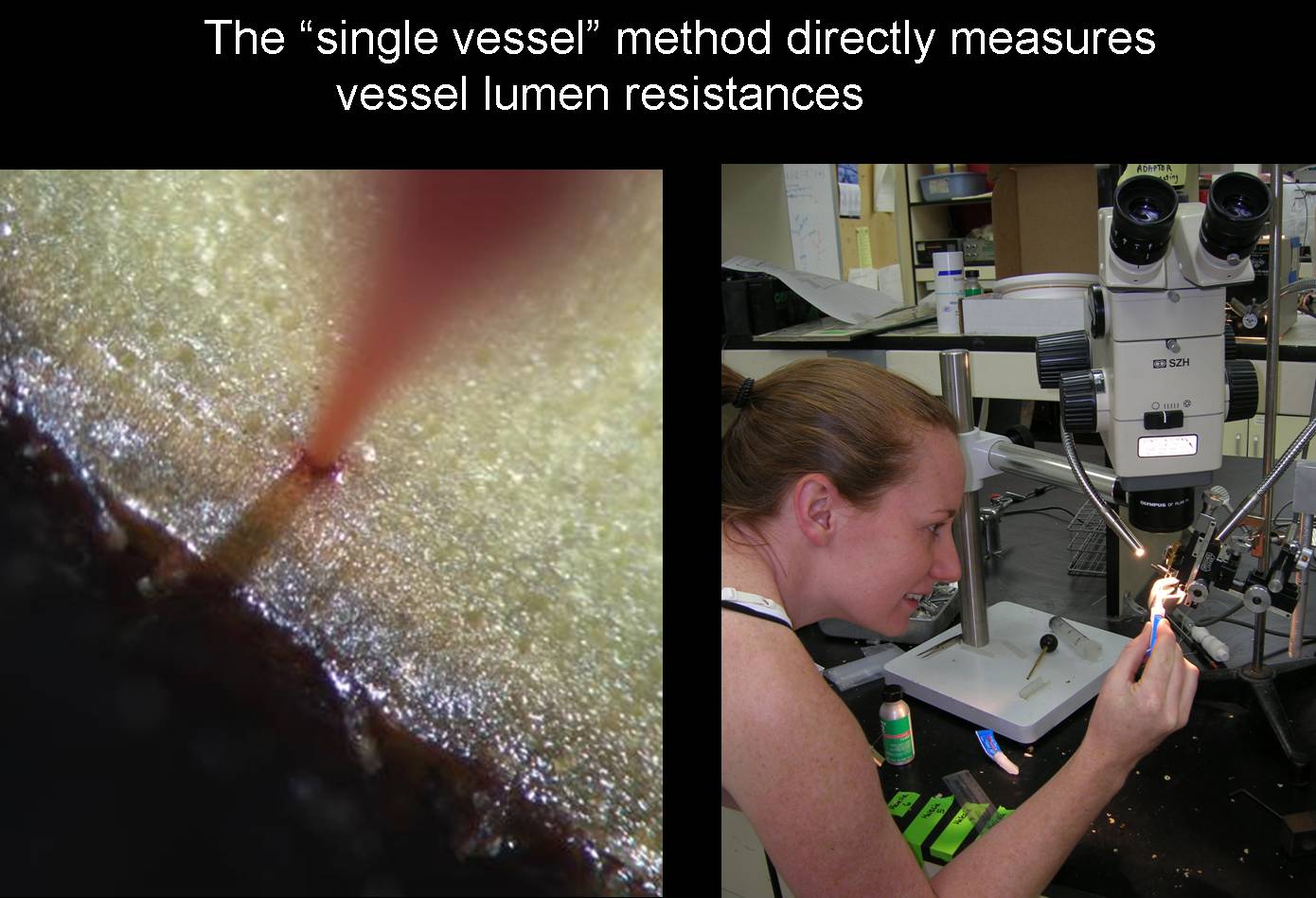
Maggie Christman employing the single-vessel technique.
Future projects in this area may involve linking the evolution of conducting capacity with growth form and habitat. In theory, adaptation to different niches involves very different demands on the water transport system. Shade-adapted plants of nutrient poor montane cloud forests have less need for efficient water transport than sun-adapted pioneer trees of nutrient rich forests. Evolution of more efficient transport, including the elimination of scalariform perforation plates, should proceed more rapidly in lineages adapting to high water demand. Testing this kind of hypothesis is on the horizon.
Hydraulic architecture and metabolic scaling theory
In theory, transport capacity should correlate with metabolic rate. We have demonstrated this experimentally with respect to net photosynthesis (link to Hubbard et al. 2001, PDF). Brian Enquist and colleagues have developed an allometric model of plant vascular transport which predicts how metabolic rate (respiration and photosynthesis) scales with size. We have recently teamed up with Brian (University of Arizona) and Van Savage (UCLA) to revise their influential model to more closely represent the anatomical and morphological diversity. Preliminary results suggest that variations in conduit taper, packing, and amount of sapwood in the tree network create a spectrum of scaling exponents rather than the single universal exponent predicted by the original model (BSA poster).
Graduate student Erica von Allmen is testing the revised model in ring-porous oaks and diffuse-porous maples. She is measuring whole tree water use in both species as a function of tree size for comparison with model predictions. Growth curves (shoot mass per year) will relate tree water use to net biomass accumulation. Previous work suggests that ring-porous trees use less water per DBH than diffuse-porous trees, but sample sizes were small and there are methodological issues. Erica's study should avoid these shortcomings and provide a robust test of the model in two types of trees with sharply contrasting vascular anatomy. New graduate student Duncan Smith has spearheaded the development of a numerical model that we are in the process of applying to trees with variable branching structure, relaxing the strictly self-similar branching of the original version.
Modeling the soil-plant-atmosphere continuum
Cavitation and soil-drying place physical limits on the rate of plant water transport. If transpiration exceeds this critical rate, hydraulic conductance of the soil-plant continuum drops to zero, and the leaves become hydraulically disconnected from the soil. Years ago we developed a model of the continuum that incorporates the effects of rhizosphere drying and xylem cavitation on setting limits to transport (Sperry et al. 1998, PDF). The model also utilizes important architectural parameters, including root depth distributions and canopy leaf area profiles. It predicts whole-plant water use as a function of the soil moisture profile, along with the maximum theoretical rate of water use. When fully parameterized, it can do a good job of matching measured plant water use and how it changes in response to drought and rain events (Hacke et al. 2000, PDF). More generally, the model provides insight into the coordination between stomatal regulation and hydraulic function, effects of soil texture and moisture profile, and the importance of root-to-leaf ratios (summary: Sperry et al. 2002, PDF).
Transpiration predicted by the model (crosses) matches measured (circles) changes iin response to variations in soil water potential (triangles). Actual water use approaches theoretical carrying capacity during drought (solid symbols).
More recently, the model has been used to understand the contrasting water use by pinyon and juniper trees which dominate the middle-elevation vegetation in much of the intermountain west (West et al. 2008, PDF). Cavitation-sensitive pinyon protects itself during drought by stomatal closure, preserving its hydraulic system for the next rain event. But stomatal closure comes at the cost of reduced photosynthesis and vulnerability to bark beetles. Cavitation-resistant juniper continues gas exchange during drought, extracting more water from the soil. But in doing so, the Juniper suffers considerable cavitation, making it less competitive for water after the next rain. Collaboration with Will Pockman (University of New Mexico) and Nate McDowell (Los Alamos) involves using the transport model to help understand the extensive mortality of pinyon pine across the southeastern US in recent years (McDowell et al. 2008, PDF; see Picture).

The transport model is being used to understand drought related mortality in pinyon-juniper woodlands.
A new collaboration with Antonio Diaz Espejo (Instituto de Recursos Naturales y Agrobiología de Sevilla, Spain) will apply the model to improvement of olive and almond cultivation. We plan to revise the model to be more user-friendly, run in non-steady-state time, and incorporate stomatal conductance and photosynthesis.

